Neural tissue interfacing lab
Discovering the impact of insertion parameters for optimizing neural yield,
biocompatibility and quality of multi-channel extracellular recordings

Strategies for seamless
probe implantation and integrationOptimizing parameters: insertion speed, probe angle, site layout, shank geometry, novel probe materials, coatings, vasoconstrictors, artefact filtering and more.
High-density neural probes allow the recording of brain electrical activity with high spatial and temporal resolution. These electrical imaging devices if implanted chronically, can be used for the long-term monitoring of the activity of single neurons to study brain mechanisms where electrophysiological changes over time are relevant (e.g. learning and memory). Furthermore, neural electrical imaging and stimulation techniques are important components of several neural prosthetic device and brain-computer interface applications.

Unfortunately, the long-term performance of most neural probes is limited, the electrical impulses (or spikes) of neurons can be recorded for days or weeks, sometimes for months, but rarely for longer periods. The scale of performance degradation may depend on numerous factors like the biocompatibility of the implanted device, the biological response of the brain tissue around the implantation site, hemorrhage by rupturing blood vessels during insertion and micro- or macromovements of the neural probe relative to the brain. These factors can significantly contribute to the damage and subsequent death of neurons and to an elevated tissue reaction resulting in the formation of a glial scar around the implanted device.

Our aim is the development of a complex implantation strategy which may contribute to achieve high-quality neural recordings for long periods of time. This strategy includes several methods to minimize, on the one hand, the tissue damage during probe insertion and on the other hand, the biological response of the brain tissue to the implanted probes. Such methods, for instance, are the local cooling of the brain tissue near the implant site, local application of vasoconstrictor substances to decrease probe insertion-related bleeding, design and 3D printing of protective components (e.g. head caps) for chronically implanted probes, or a careful design of the insertion conditions (e.g. using a very slow insertion speed).
Furthermore, we are investigating novel probe materials and coatings which may enhance the biocompatibility of the implanted devices which in turn may provide better quality neural signals and for a longer term.
Members
- István Ulbert, MD, DSc (Full professor, head of the lab, group leader)
- Lucia Wittner, DSc (Full professor)
- Richárd Fiáth, PhD (Senior research associate)
- Gergely Márton, PhD (Senior research associate)
- Kinga Tóth, PhD (Research associate)
- Domonkos Horváth, PhD (Research associate)
- Domokos Meszéna, PhD (Research associate)
- Csaba Horváth, MSc (PhD candidate)


Lab Facilities
- ATLASNeuro silicon probes (http://www.atlasneuro.com/)
- Intan RHD2000 Evaluation System for high-density recording
(http://intantech.com/RHD2000_evaluation_system.html) - NeuroNexus silicon probes (https://neuronexus.com/)
- Neuropixels silicon probes (https://www.neuropixels.org/)
- High-density (128 and 256) channel passive silicon probes (http://www.neuroseeker.eu/)
- High-density active CMOS probes (http://www.neuroseeker.eu/)
- Neurostar Robot Stereotaxic (https://neurostar.de/robot-stereotaxic/)
- Kopf Small Animal Stereotaxic (https://kopfinstruments.com/)
- Xilinx Kintex-7 FPGA KC705 Evaluation Kit
(https://www.xilinx.com/products/boards-and-kits/ek-k7-kc705-g.html) - In vitro setup (linear multiple channel microelectrode + sharp intracellular)
- Leica Vibratome 1000S + 1200 VT
- Two-photon laser scanning microscope (Femtonics/Olympus)
- Coherent Chameleon Ultra II femtosecond pulse lasers (galvo and resonant)
- Leica light microscope with fluorescence
- Jeol electron microscope (external, at University of Veterinary Medicine, Budapest)


Team publications
Selected publications
Please visit our Publications page for the full list.
- Fiáth R, Márton AL, Mátyás F, Pinke D, Márton G, Tóth K, Ulbert I. Slow insertion of silicon probes improves the quality of acute neuronal recordings. (2019) SCIENTIFIC REPORTS 9: Paper 111. IF: 4.122, Q1/D1
- Meszéna D, Kerekes BP, Pál I, Orbán G, Fiáth R, Holzhammer T, Ruther P, Ulbert I and Márton G. A silicon-based spiky probe providing improved cell accessibility for in vitro brain slice recordings. SENSORS & ACTUATORS B – CHEMICAL, 126649, 2019. IF: 6.39, Q1/D1
- Zátonyi A, Orbán G, Modi R, Márton G, Meszéna D, Ulbert I, Pongrácz A, Ecker M, Voit WE, Joshi-Imre A, Fekete Z. A softening laminar electrode for recording single unit activity from the rat hippocampus. (2019) SCIENTIFIC REPORTS 9: 2321. IF: 4.122, Q1/D1
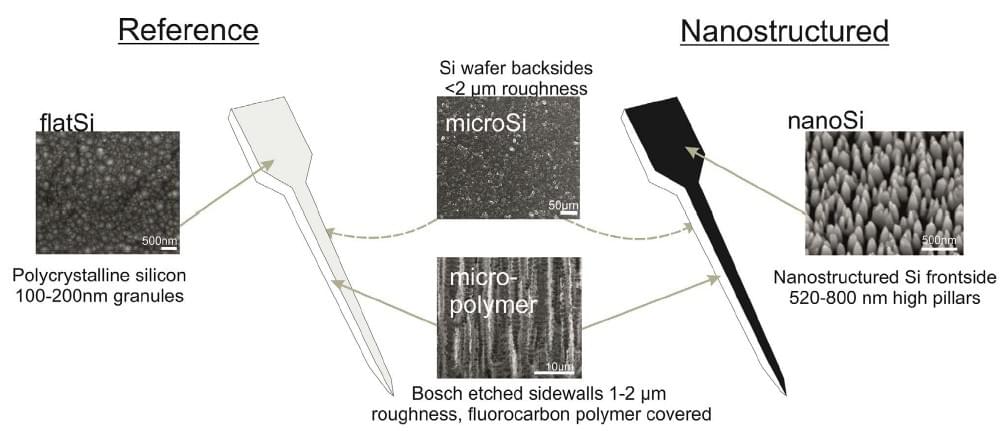
- Zs Bérces, K Tóth, G Márton, I Pál, B Kováts-Megyesi, Z Fekete, I Ulbert, A Pongrácz. Neurobiochemical changes in the vicinity of a nanostructured neural implant. (2016) SCIENTIFIC REPORTS 6: Paper 35944. IF: 5.228, Q1/D1
- Fekete Z, Nemeth A, Marton G, Ulbert I and Pongracz A. Experimental study on the mechanical interaction between silicon neural microprobes and rat dura mater during insertion. (2015) JOURNAL OF MATERIALS SCIENCE: MATERIALS IN MEDICINE 26(2): 70. IF: 2.325, Q2
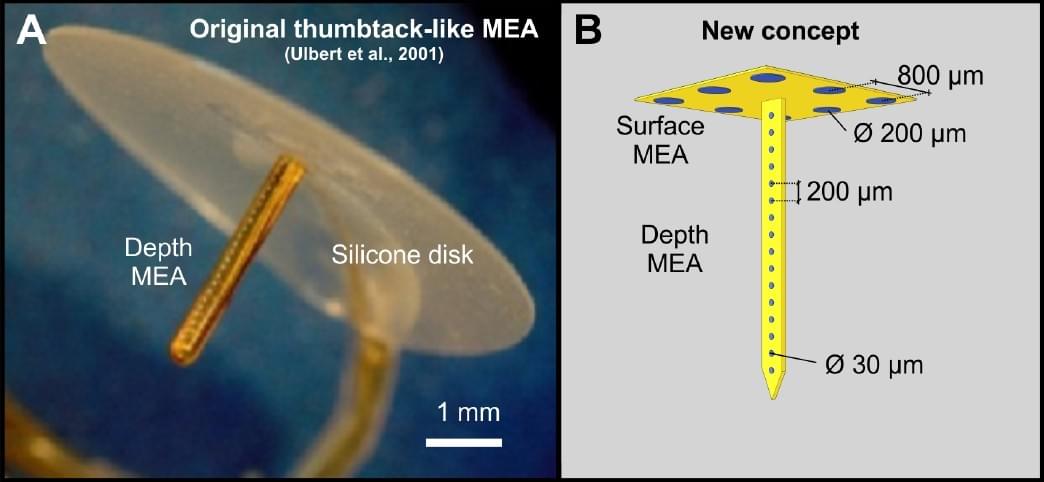
- G Márton, G Orbán, M Kiss, R Fiáth, A Pongrácz, I Ulbert: A multimodal, SU-8 – platinum – polyimide microelectrode array for chronic in vivo neurophysiology. (2015) PLOS ONE.,10(12), e0145307. IF: 3,234, Q1
- Fiáth R, Márton AL, Mátyás F, Pinke D, Márton G, Tóth K, Ulbert I. Slow insertion of silicon probes improves the quality of acute neuronal recordings. (2019) SCIENTIFIC REPORTS 9: Paper 111. IF: 4.122, Q1/D1
© 2024 Ulbert | Lab